Clinical Research Associate (East Coast - US Region)
- St Joseph Hospital & Medical Center
- Phoenix, Arizona, Remote
About Us
Inspired by faith. Driven by innovation. Powered by humankindness. CommonSpirit Health is building a healthier future for all through its integrated health services. As one of the nation’s largest nonprofit Catholic healthcare organizations, CommonSpirit Health delivers more than 20 million patient encounters annually through more than 2,300 clinics, care sites and 138 hospital-based locations, in addition to its home-based services and virtual care offerings.
Our Mission
As CommonSpirit Health, we make the healing presence of God known in our world by improving the health of the people we serve, especially those who are vulnerable, while we advance social justice for all. To learn more about a calling that defines and unites, please click here for more information about our mission, vision, and values.
The posted compensation range of $35.62 - $52.99 /hour is a reasonable estimate that extends from the lowest to the highest pay CommonSpirit in good faith believes it might pay for this particular job, based on the circumstances at the time of posting. CommonSpirit may ultimately pay more or less than the posted range as permitted by law.
- Job ID
- 2026-456220
- Employment Type
- Full Time
- Department
- Research - Cro Activities
- Hours/Pay Period
- 80
- Weekly Schedule
- Monday - Friday (8:00 AM - 5:00 PM)
- Shift
- Day
- Remote
- Yes
- Category
- Research
Job Summary and Responsibilities
Join BNI CRO as a Clinical Research Associate (CRA) and collaborate with dedicated research sites, contributing to rare neurological disease research. Make a significant impact in the scientific community alongside a dedicated team of CRAs. This remote position involves up to 75% travel across the US, primarily focusing on northeast and east coast regional site assignments. Your role will encompass important site monitoring and management activities, including SIVs, Interim, and Remote Monitoring Visits, as well as Final Onsite and Closeout Visits. Advance your career, and acquire valuable skills at Barrow Neurological Institute, all while contributing to groundbreaking clinical research!
This Clinical Research Associate will report directly to the BNI Clinical Research Organization (CRO) Program Manager. The CRA position will independently be responsible for clinical monitoring activities and overall project administration to oversee the progress of multi-center, investigator initiated, or industry sponsored clinical studies throughout the United States and Canada. The CRA will travel to assigned clinical research sites to conduct approx. 25-30 clinical trial monitoring visits each year, and will also complete remote/centralized monitoring visits to ensure clinical studies are conducted, recorded, and reported in accordance with the protocol, standard operating procedures (SOPs), International Conference on Harmonization- Good Clinical Practice (ICH-GCP) guidelines, and all applicable regulatory requirements.
The CRA will be responsible for the timely reporting of all monitoring visit findings, and is expected to complete comprehensive monitoring reports and associated deliverables to provide back to the Sponsor and the clinical sites. Additionally, the CRA role will oversee clinical site training activities and, conduct remote webinar and onsite protocol training for Physician Site Investigators, Clinical Research Coordinators, Research Nurses, and other delegated healthcare study personnel. The CRA will be responsible for managing all monitoring visit travel arrangements and travel expense reporting activities in accordance with Dignity Health’s travel and expense purchasing policy. The CRA role will actively participate in departmental quality control and program development activities as directed by the BNI CRO Program Manager.
Responsibilities Include:
- Responsible for all aspects of clinical monitoring, site management, and trial administration as prescribed in the monitoring plan and as instructed by program leadership. Employee is responsible for working with sites during study start up, including assessing site feasibility as required, provide ongoing training and site initiation visits, conducting remote monitoring, and approx. 25-30 interim onsite and closeout field monitoring visits located throughout the US and Canada. During each monitoring visit the CRA is responsible for the evaluation of clinical data documentation, regulatory document review, monitor safety and conduct of study to ensure investigator and site compliance with study protocol, overall clinical objectives, FDA regulations, ICH/GCP guidelines, institutional/ CRO SOPs, and HIPAA guidelines. The CRA is expected to work cooperatively with study team and operations management to proactively drive project success.
- Completes preparation and submission of accurate and timely monitoring reports, and other requested deliverables, as outlined in the Study Monitoring Plan or as requested by program leadership.
- Effective long-term planning/scheduling of remote and on-site monitoring visits in accordance with the Study Monitoring Plan or as requested by program leadership.
- Perform Lead Study Monitoring activities as assigned, including acting as the primary liaison between BNI CRO and the Clinical Study Sponsor to ensure BNI CRO provides quality monitoring and site management customer services. The Lead Study Monitor will be responsible for the timely review of all monitoring deliverables, conduct co-monitoring visits and mentorship of CRA team members.
Job Requirements
Minimum Required
2-3 years of clinical research experience required or a combination of equivalent experience and education.
Excellent written /oral communication, interpersonal, and organizational skills required.
Good presentation skills and ability to effectively communicate information and processes to a variety of audiences.
Ability to travel frequently on domestic and extended trips throughout the United States and Canada (up to 75% of the time), to perform clinical monitoring visits, primarily in the northeast/east coast region,
Proven attention to detail, excellent problem solving and customer service skills, and ability to multi-task.
Education
Bachelors Degree or equivalent combination of education and experience or its international equivalent in clinical, science, or health related field from an accredited institution required or a healthcare professional licensure such as a Registered Nurse or Physical Therapist.
Special Skills
Strong interpersonal skills to effectively navigate a matrix organizational structure and effectively lead cross functional teams.
Excellent communication skills (verbal and written) to effectively communicate complex information and processes to a variety of audiences.
Proven attention to detail, with strong analytical, critical thinking, problem solving, time management, and customer service skills.
Experience with Microsoft Office Suite Systems
Preferred:
Electronic Data Capture System (EDC) skills preferred
CCRA or CCRP certification preferred
Located conveniently in the heart of Phoenix, Arizona,St. Joseph's Hospital and Medical Center is a 571-bed, not-for-profit hospital that provides a wide range of health, social and support services. Founded in 1895 by the Sisters of Mercy, St. Joseph's was the first hospital in the Phoenix area. More than 125 years later, St. Joseph's remains dedicated to its mission of caring for the poor and underserved.
We are extremely proud to be a nationally recognized center for quality quaternary care, medical education and research. St. Joseph's includes the internationally renowned Barrow Neurological Institute, Norton Thoracic Institute, Cancer Center at St. Joseph's, Ivy Brain Tumor Center, and St. Joseph's Level I Trauma Center (which is verified by the American College of Surgeons). The hospital is also a respected center for high-risk obstetrics, neuro-rehabilitation, orthopedics, and other medical services. St. Joseph’s is considered a sought-after destination hospital for treating the most complex cases from throughout the world. Every day, approximately 20 percent of the hospital’s patients have traveled from outside of Arizona and the United States to seek treatment at St. Joseph’s.
U.S News & World Report routinely ranks St. Joseph's among the top hospitals in the United States for neurology and neurosurgery. In addition, St. Joseph's boasts the Creighton University School of Medicine at St. Joseph's, and a strategic alliance with Phoenix Children's Hospital.
St. Joseph's is consistently named an outstanding place to work and one of Arizona's healthiest employers. Come grow your career with one of Arizona's Most Admired Companies.
Look for us on Facebookand follow us on Twitter.
For the health of our community ... we are proud to be a tobacco-free campus.
Total RewardsDepending on the position offered, CommonSpirit Health offers a generous benefit package, including but not limited to medical, prescription drug, dental, vision plans, life insurance, paid time off (full-time benefit eligible team members may receive a minimum of 14 paid time off days, including holidays annually), tuition reimbursement, retirement plan benefit(s) including, but not limited to, 401(k), 403(b), and other defined benefits offerings, as may be amended from time to time. For more information, please visit our Total Rewards
Unless directed by a Collective Bargaining Agreement, applications for this position will be considered on a rolling basis. CommonSpirit Health cannot anticipate the date by which a successful candidate may be identified.

-
Rad Tech Intern Cardiac Cath Lab
- CHI St Alexius Bismarck
- Bismarck, North Dakota
-
Nurse PCU I
- CHI Mercy Health of Roseburg
- Roseburg, Oregon
-
Nurse PCU I
- CHI Mercy Health of Roseburg
- Roseburg, Oregon
-
Nurse Hospice
- CHI Mercy Health of Roseburg
- Roseburg, Oregon
You have not viewed any jobs yet.
You have not saved any jobs yet.
Related Content
-

News & Stories at CommonSpirit Health
Visit our News & Stories page and learn more about working at CommonSpirit Health, one of the largest nonprofit health systems in the U.S.
Learn More at News & Stories at CommonSpirit Health page -

-

-

Page Title 1
Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
Learn More at Page Title 1 page -

Page Title 2
Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
Learn More at Page Title 2 page -

Page Title 3
Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
Learn More at Page Title 3 page -

Administrative Fellowship Program
The CommonSpirit Health Administrative Fellowship is a national 18-month system-wide fellowship that offers experiential learning and exposure to strategic and operational aspects of an integrated healthcare system.
Learn More at Administrative Fellowship Program page -

Lead with humankindness - Executive Positions
Meaningful work takes a village, but inspired leadership gets it done. At CommonSpirit, our leaders influence by example and succeed through accountability. In character and conduct, you embody humankindness—by pushing us toward our best selves so we can do our best work every day.
Learn More at Lead with humankindness - Executive Positions page -

Career FAQs
Have questions about our hiring process? We're here to assist you if you're ready to apply for a position with CommonSpirit Health.
Learn More at Career FAQs page -

Our Mission and Vision
Learn about CommonSpirit Health's mission, vision and values that guide our work culture and shape our commitment to delivering humankindness.
Learn More at Our Mission and Vision page -

Military
At CommonSpirit Health, we deeply value the sacrifices made by our veterans and their families. Our commitment to supporting those who served extends beyond words—reflected in the opportunities and benefits we provide.
Learn More at Military page -

Apprenticeship Programs at CommonSpirit Health
Launch your healthcare career through our apprenticeship programs, where you can earn while you learn and gain hands-on training for a successful professional role.
Learn More at Apprenticeship Programs at CommonSpirit Health page -

Connect with a Vet
Through our Connect With a Vet program, military community members can connect one-on-one with CommonSpirit Health veterans and military spouses for personal insights into our culture, work environment, and daily life at our organization.
Learn More at Connect with a Vet page -

Interventional Radiology | CommonSpirit Health
Learn More at Interventional Radiology | CommonSpirit Health page -

National Real Estate Services at CommonSpirit Health
Explore career opportunities in real estate strategy, property management, facilities management, design and construction, and support services at CommonSpirit Health's National Real Estate Services team.
Learn More at National Real Estate Services at CommonSpirit Health page -

Our Benefits
Our benefits and perks are a big part of why we’re ranked a top employer.
Learn More at Our Benefits page -

Diversity & Inclusion
At CommonSpirit, we are dedicated to delivering humankindness. Diversity is not just an initiative, it’s the true nature of who we are.
Learn More at Diversity & Inclusion page -

Nursing
Explore more about nursing opportunities, our nursing vision and hear from our staff members.
Learn More at Nursing page -

Recent Graduate Jobs at CommonSpirit Health
Earn top-of-class work experience with a nursing leader. As a graduate with the pedigree, tech-savvy, and faith in your own destiny, you embody the next generation of healthcare. At CommonSpirit, our investment in you, personally and professionally, is how we achieve our mission, vision, and values.
Learn More at Recent Graduate Jobs at CommonSpirit Health page -

CommonSpirit Health teams up with Proxima Careers
CommonSpirit Health and Proxima Careers are partnering to expand access to healthcare careers by providing training, job placement, and ongoing support, helping community members achieve stable, well-paying jobs.
Learn More at CommonSpirit Health teams up with Proxima Careers page -

CommonSpirit Health Alumni Community
Interested in returning to CommonSpirit Health? Learn about jobs, company news, and hear from our team members.
Learn More at CommonSpirit Health Alumni Community page -

Application Notices and Information
Learn about CommonSpirit Health's application process, important notices, and requirements.
Learn More at Application Notices and Information page -

CHI Health 2026 Nursing Internship
CHI Health Nursing Internship in Nebraska
Learn More at CHI Health 2026 Nursing Internship page -

CHI Health Career Ladder CNA Program
CHI Health has partnered with local Certified Nursing Assistant Programs to offer students C.N.A. training in exchange for full or part-time employment!
Learn More at CHI Health Career Ladder CNA Program page -

Innovative Virtual Nursing Model
CommonSpirit has two types of virtual nurses: ones who work at a command center to help with admissions, discharges and transfers, and ones who are part of the care team, attending rounds with physicians and being available to patients at the push of the button.
Learn More at Innovative Virtual Nursing Model page -

-

Make an impact in Therapy at CommonSpirit Health
Explore the wide range of therapy opportunities available at CommonSpirit Health, a mission-driven health system dedicated to improving lives through compassionate, relationship-based care.
Learn More at Make an impact in Therapy at CommonSpirit Health page -

-

CommonSpirit Health | Stepful
CommonSpirit Health is proud to partner with Stepful to connect skilled, motivated students with rewarding Allied Health career opportunities.
Learn More at CommonSpirit Health | Stepful page -

-

COVID-19 Vaccination Requirements
COVID-19 Vaccination Requirements at CommonSpirit Health
Learn More at COVID-19 Vaccination Requirements page -

Clinical Engineering Careers at CommonSpirit Health
A career in clinical engineering offers a fulfilling blend of technology and healthcare, with the potential to make a positive impact on patient outcomes and the overall efficiency of healthcare delivery. Learn more and explore a career in clinical engineering at CommonSpirit Health.
Learn More at Clinical Engineering Careers at CommonSpirit Health page -

Careers in Environmental Services at CommonSpirit Health
Explore career opportunities in Environmental Services at CommonSpirit Health, where we provide a clean and safe environment for patients, staff, and visitors.
Learn More at Careers in Environmental Services at CommonSpirit Health page -

Radiology Careers at CommonSpirit Health
Discover fulfilling career opportunities in Imaging Services at CommonSpirit Health and play a vital role in the diagnosis and treatment of patients' care plans.
Learn More at Radiology Careers at CommonSpirit Health page -

Pursue Your Path as a Medical Assistant at CommonSpirit Health
As a Medical Assistant at CommonSpirit Health, you'll be a crucial part of the healthcare system, making a tangible difference in patients' lives. Explore this rewarding career opportunity.
Learn More at Pursue Your Path as a Medical Assistant at CommonSpirit Health page -

Elevate your career as a Surgical Tech with CommonSpirit Health
As a surgical technician at CommonSpirit Health, you will play a vital role in guaranteeing that surgical procedures are safe and successful. You will collaborate with leading surgeons and healthcare experts in cutting-edge facilities, providing our patients with the utmost care.
Learn More at Elevate your career as a Surgical Tech with CommonSpirit Health page -

Administrative Fellowship Operations Track
The CommonSpirit Health Administrative Fellowship Operations Track will provide fellows with a broad range of health care experiences at the national and local level.
Learn More at Administrative Fellowship Operations Track page -

CommonSpirit is Advancing Equitable Opportunities for Hiring and Training
CommonSpirit Health is engaged in the Healthcare Anchor Network’s Impact Workforce Commitment, an initiative to hire and train individuals who may have faced employment barriers due to low income, lack of access to education and training, and other socioeconomic challenges.
Learn More at CommonSpirit is Advancing Equitable Opportunities for Hiring and Training page -

CommonSpirit Health Hospital Medicine
Practicing hospital medicine at CommonSpirit Health offers a unique chance to serve a mission-driven organization dedicated to improving health in diverse communities. Physicians thrive in a collaborative, interdisciplinary environment that supports holistic care, innovation, and continuous growth. With a strong focus on equity and access, hospitalists help meet the needs of underserved populations and make a lasting impact.
Learn More at CommonSpirit Health Hospital Medicine page -

CHI Health Locations
Now is the perfect time to join our CHI team in one of our locations!
Learn More at CHI Health Locations page -

Los Angeles County COVID Requirements
CommonSpirit Health Locations with COVID/FLU Vaccincation Requirements
Learn More at Los Angeles County COVID Requirements page -

Meet the CommonSpirit Health Fellows
Meet our current CommonSpirit Health Fellows. This is a national, 18-month, system-wide leadership and professional development program that offers a solid foundation for future leaders through hands-on learning experiences, exposure to clinical and non-clinical aspects and mentoring from influential innovators.
Learn More at Meet the CommonSpirit Health Fellows page -

CommonSpirit Mountain Region New Grad RN Residency
Start your nursing career with our evidence-based New Grad RN Residency Program. Receive comprehensive training and support to become the nursing professional you've envisioned.
Learn More at CommonSpirit Mountain Region New Grad RN Residency page -

National Nursing New Grad Residency Program
Learn about our new grad nursing program that is nationally-standardized and evidence-based best practice for newly licensed nurses
Learn More at National Nursing New Grad Residency Program page -

Online Accessibility Notice
Online Accessibility Notice
Learn More at Online Accessibility Notice page -

Learn about our National Travel Program
Join our National Travel Program and enjoy the variety of a travel caregiver with the security of being employed by one of the largest faith-based healthcare systems in the country.
Learn More at Learn about our National Travel Program page -

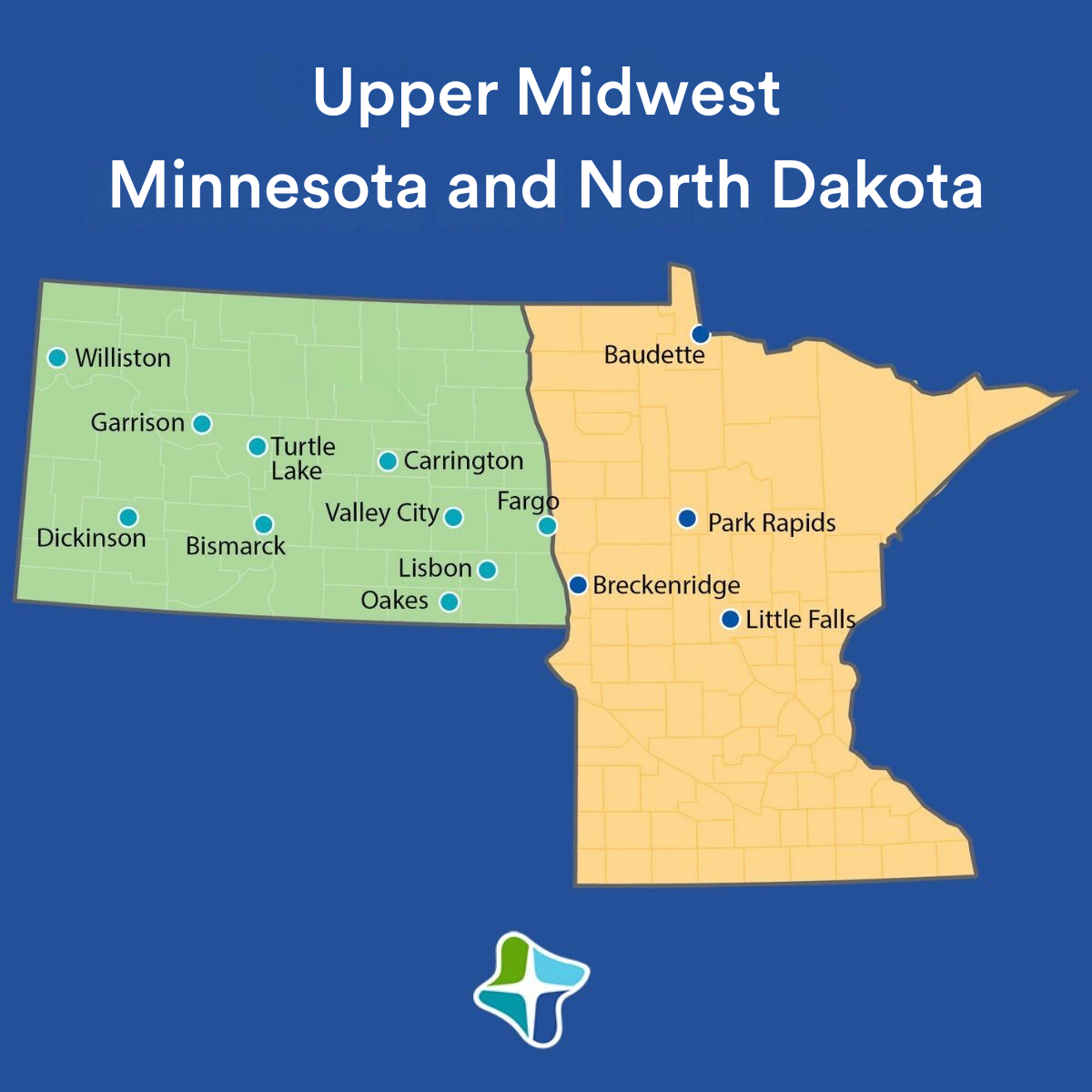
Upper Midwest
Now is the perfect time to join our healthcare team in one of our Upper Midwest locations in Minnesota and North Dakota!
Learn More at Upper Midwest page -

Learn about our Virtually Integrated Nursing Care Model
Virtually Integrated Care provides assistance to the bedside team and patient/family through the use of virtual technology.
Learn More at Learn about our Virtually Integrated Nursing Care Model page -

Virtual Command Center
Ever wondered what it was like to work in a Virtual Command Center? We’re in the background continuously assessing, reviewing and providing interventions to patients at all hours of the day across the nation.
Learn More at Virtual Command Center page -

On Demand Staffing
On Demand Staffing at CommonSpirit Health offers flexible schedules for nurses.
Learn More at On Demand Staffing page




























Share Job