Research Data Coordinator
The posted compensation range of $25.55 - $38 /hour is a reasonable estimate that extends from the lowest to the highest pay CommonSpirit in good faith believes it might pay for this particular job, based on the circumstances at the time of posting. CommonSpirit may ultimately pay more or less than the posted range as permitted by law.
Responsibilities
The Ivy Brain Tumor Center is a non-conventional non-profit program offering state-of-the-art clinical trials for patients with the most aggressive form of brain cancer. It is home to the largest collection of Phase 0 trials for brain tumors in the world. Our multidisciplinary team of brain tumor specialists and scientists are accelerating drug discovery and precision medicine in neuro-oncology to identify the most promising first-in-class therapies for brain tumor patients. Our day-to-day interactions with patients caregivers and each other are grounded in transparency: our conversations are always two-way and our labs are open to ensure efficient collaboration. Everyone at the Ivy Brain Tumor Center is passionate about identifying and accelerating treatments that will make a meaningful difference in patients’ lives. Here innovation never stops – because for us this work is personal.
Visit us at Ivybraintumorcenter.org, and follow us on Facebook, Twitter, Instagram, LinkedIn and YouTube
The Research Data Coordinator (RDC) is a highly skilled role that autonomously reviews and synthesizes information from medical records (clinic notes pathology reports radiology reports patient questionnaires etc.) and source documents to extract industry cooperative and investigator-initiated trial data as specified by assigned research protocols. The position is responsible for protocol-specific data requirements for multiple complex research projects while ensuring data requirements are in alignment with good clinical practice according to Federal regulations.
Principal Duties and Responsibilities:
- Maintains a high-level of expertise and abstracts information independently from a wide range of data collection tools or the subject's medical record and enters required protocol data into the study-designated data collection system
- Ensures data is entered/reported in a timely manner keeping with clinical trial agreements and institutional standard operating procedures.
- Identifies data discrepancies and/or missing data and follows up with investigators and the study team to resolve.
- Reports data quality issues at study meetings and provides suggestions for ways to resolve the issue
- Reviews monitoring/audit reports database queries and resolve errors to ensure the integrity of data.
- Provides coverage for the team as appropriate.
- Responsible for reporting on each study including information related to protocol activity data monitoring/auditing and other research information; presents this information at regular research staff meetings.
- Ensure data compliance for ICH GCP and regulatory authorities.
- Develops methods for tracking and reporting data and monitors incoming and outgoing data to ensure data integrity and compliance with applicable regulatory agency standards.
- May support the lead data management specialist in the development of the study database according to clinical trial specifications including: eCRF design user requirements edit check specifications user acceptance testing (UAT) medical coding query management data validation programming and testing data imports/exports.
- Ensure data system compliance for ICH GCP and regulatory authorities.
- May train others in work responsibilities.
- Collaborates with the investigator(s) study coordinator research nurse monitors and data manager regarding the protocol requirements.
- Collaborates with the study coordinator research nurse or research assistant to document and report study patient enrollment treatment and follow-up into the study database including protocol adherence adverse events and treatment outcomes.
- Assists with the coordination of organized meetings conference calls and training.
- Participates in site initiation interim monitoring and close-out visits internal audits and external audits/inspections.
- Attends and actively participates in regularly scheduled multidisciplinary tumor boards staff meetings disease site program research meetings and role-specific meetings.
- Participates in department-based process improvement or advisory groups.
Qualifications
Required Qualifications: A minimum of a HS diploma and 1 year of clinical research/ experience; or equivalent combination of education training and experience.
Preferred Qualifications/Skills:
Strong medical terminology understanding Certification in Clinical Research preferred (e.g. CCRC CCRP) Strong knowledge of Clinical Practice (GCP) and International Conference on Harmonization (ICH) guidelines Proven ability to work with others to deliver results to the appropriate quality and timeline metrics Must possess effective verbal and written communication skills as well as excellent interpersonal skills with staff and other healthcare professionals Must have the ability to prioritize and pace oneself when working under the pressure of deadlines and work volume Previous experience taking place in the oncology research setting preferred Thorough knowledge of EMR systems (e.g. Cerner) clinical trial management systems (e.g RealTime) or other data entry systems (e.g. RedCap Medidata Rave)
High School Graduate or GED upon hire and1 year of clinical research/experience; or equivalent combination of education,training, and experience required.
Previous experience taking place in the oncology research setting preferred
Bachelors degree preferred
Preferred Licensure and Certifications:
Clinical Research Associate (CCRA)
Certified Clinical Research Coordinator ACRP (CCRC-ACRP)
Certified Clinical Resarch Professional SOCRA (CCRP-SOCRA)
Additional Requirements (e.g. two letters of recommendation)
OverviewHello humankindness Located conveniently in the heart of Phoenix, Arizona,St. Joseph's Hospital and Medical Center is a 571-bed, not-for-profit hospital that provides a wide range of health, social and support services. Founded in 1895 by the Sisters of Mercy, St. Joseph's was the first hospital in the Phoenix area. More than 125 years later, St. Joseph's remains dedicated to its mission of caring for the poor and underserved. We are extremely proud to be a nationally recognized center for quality quaternary care, medical education and research. St. Joseph's includes the internationally renowned Barrow Neurological Institute, Norton Thoracic Institute, Cancer Center at St. Joseph's, Ivy Brain Tumor Center, and St. Joseph's Level I Trauma Center (which is verified by the American College of Surgeons).
The hospital is also a respected center for high-risk obstetrics, neuro-rehabilitation, orthopedics, and other medical services. St. Joseph’s is considered a sought-after destination hospital for treating the most complex cases from throughout the world. Every day, approximately 20 percent of the hospital’s patients have traveled from outside of Arizona and the United States to seek treatment at St. Joseph’s. U.S News & World Report routinely ranks St. Joseph's among the top hospitals in the United States for neurology and neurosurgery. In addition, St. Joseph's boasts the Creighton University School of Medicine at St. Joseph's, and a strategic alliance with Phoenix Children's Hospital. St. Joseph's is consistently named an outstanding place to work and one of Arizona's healthiest employers.
Come grow your career with one of Arizona's Most Admired Companies.
Look for us on Facebook and follow us on Twitter. For the health of our community ... we are proud to be a tobacco-free campus.
Unless directed by a Collective Bargaining Agreement, applications for this position will be considered on a rolling basis. CommonSpirit Health cannot anticipate the date by which a successful candidate may be identified.
Apply
Depending on the position offered, CommonSpirit Health offers a generous benefit package, including but not limited to medical, prescription drug, dental, vision plans, life insurance, paid time off (full-time benefit eligible employees may receive a minimum of 14 paid time off days, including holidays annually), tuition reimbursement, retirement plan benefit(s) including, but not limited to, 401(k), 403(b), and other defined benefits offerings, as may be amended from time to time. For more information, please visit https://www.commonspirit.careers/benefits.
No featured jobs
No recently viewed jobs
You have no saved jobs
-
 Explore Hiring Events at CommonSpirit Health Explore career opportunities at CommonSpirit Health, a leading healthcare organization committed to delivering humankindness. Browse open positions and learn about our mission, values, and benefits.
Explore Hiring Events at CommonSpirit Health Explore career opportunities at CommonSpirit Health, a leading healthcare organization committed to delivering humankindness. Browse open positions and learn about our mission, values, and benefits. -
 Our Mission and Vision Learn about CommonSpirit Health's mission, vision and values that guide our work culture and shape our commitment to delivering humankindness.
Our Mission and Vision Learn about CommonSpirit Health's mission, vision and values that guide our work culture and shape our commitment to delivering humankindness. -
 Diversity & Inclusion At CommonSpirit, we are dedicated to delivering humankindness. Diversity is not just an initiative, it’s the true nature of who we are. Related Content
Diversity & Inclusion At CommonSpirit, we are dedicated to delivering humankindness. Diversity is not just an initiative, it’s the true nature of who we are. Related Content -
 About Us Working at Commonspirit
About Us Working at Commonspirit -
 Lead with humankindness - Executive Positions Meaningful work takes a village, but inspired leadership gets it done. At CommonSpirit, our leaders influence by example and succeed through accountability. In character and conduct, you embody humankindness—by pushing us toward our best selves so we can do our best work every day.
Lead with humankindness - Executive Positions Meaningful work takes a village, but inspired leadership gets it done. At CommonSpirit, our leaders influence by example and succeed through accountability. In character and conduct, you embody humankindness—by pushing us toward our best selves so we can do our best work every day. -
 Nursing Explore more about nursing opportunities, our nursing vision and hear from our staff members. Related Content
Nursing Explore more about nursing opportunities, our nursing vision and hear from our staff members. Related Content -
 Our Benefits Our benefits and perks are a big part of why we’re ranked a top employer. Related Content
Our Benefits Our benefits and perks are a big part of why we’re ranked a top employer. Related Content -
 Administrative Fellowship Program The CommonSpirit Health Administrative Fellowship is a national 18-month system-wide fellowship that offers experiential learning and exposure to strategic and operational aspects of an integrated healthcare system.
Administrative Fellowship Program The CommonSpirit Health Administrative Fellowship is a national 18-month system-wide fellowship that offers experiential learning and exposure to strategic and operational aspects of an integrated healthcare system. -
 Recent Graduate Jobs at CommonSpirit Health Earn top-of-class work experience with a nursing leader. As a graduate with the pedigree, tech-savvy, and faith in your own destiny, you embody the next generation of healthcare. At CommonSpirit, our investment in you, personally and professionally, is how we achieve our mission, vision, and values.
Recent Graduate Jobs at CommonSpirit Health Earn top-of-class work experience with a nursing leader. As a graduate with the pedigree, tech-savvy, and faith in your own destiny, you embody the next generation of healthcare. At CommonSpirit, our investment in you, personally and professionally, is how we achieve our mission, vision, and values. -
 Career FAQs Have questions about our hiring process? We're here to assist you if you're ready to apply for a position with CommonSpirit Health.
Career FAQs Have questions about our hiring process? We're here to assist you if you're ready to apply for a position with CommonSpirit Health. -
 Online Accessibility Notice Online Accessibility Notice
Online Accessibility Notice Online Accessibility Notice -
 Copyrights Copyrights
Copyrights Copyrights -
 Application Notices and Information Learn about CommonSpirit Health's application process, important notices, and requirements. Hiring Process
Application Notices and Information Learn about CommonSpirit Health's application process, important notices, and requirements. Hiring Process -
 CommonSpirit Health Alumni Community Interested in returning to CommonSpirit Health? Learn about jobs, company news, and hear from our team members. Hiring Process Blog
CommonSpirit Health Alumni Community Interested in returning to CommonSpirit Health? Learn about jobs, company news, and hear from our team members. Hiring Process Blog -
 Administrative Fellowship Operations Track The CommonSpirit Health Administrative Fellowship Operations Track will provide fellows with a broad range of health care experiences at the national and local level.
Administrative Fellowship Operations Track The CommonSpirit Health Administrative Fellowship Operations Track will provide fellows with a broad range of health care experiences at the national and local level. -
 COVID-19 Vaccination Requirements COVID-19 Vaccination Requirements at CommonSpirit Health
COVID-19 Vaccination Requirements COVID-19 Vaccination Requirements at CommonSpirit Health -
 Clinical Engineering Careers at CommonSpirit Health A career in clinical engineering offers a fulfilling blend of technology and healthcare, with the potential to make a positive impact on patient outcomes and the overall efficiency of healthcare delivery. Learn more and explore a career in clinical engineering at CommonSpirit Health. Hiring Process Blog
Clinical Engineering Careers at CommonSpirit Health A career in clinical engineering offers a fulfilling blend of technology and healthcare, with the potential to make a positive impact on patient outcomes and the overall efficiency of healthcare delivery. Learn more and explore a career in clinical engineering at CommonSpirit Health. Hiring Process Blog -
 Learn about our National Travel Nurse Program Join our National Travel Nursing Program and enjoy the variety of a travel nurse with the security of being employed by one of the largest faith-based healthcare systems in the country.
Learn about our National Travel Nurse Program Join our National Travel Nursing Program and enjoy the variety of a travel nurse with the security of being employed by one of the largest faith-based healthcare systems in the country. -
 Learn about our Virtually Integrated Nursing Care Model Virtually Integrated Care provides assistance to the bedside team and patient/family through the use of virtual technology.
Learn about our Virtually Integrated Nursing Care Model Virtually Integrated Care provides assistance to the bedside team and patient/family through the use of virtual technology. -
 Innovative Virtual Nursing Model CommonSpirit has two types of virtual nurses: ones who work at a command center to help with admissions, discharges and transfers, and ones who are part of the care team, attending rounds with physicians and being available to patients at the push of the button. Hiring Process Blog
Innovative Virtual Nursing Model CommonSpirit has two types of virtual nurses: ones who work at a command center to help with admissions, discharges and transfers, and ones who are part of the care team, attending rounds with physicians and being available to patients at the push of the button. Hiring Process Blog -
 Military At CommonSpirit Health, we deeply value the sacrifices made by our veterans and their families. Our commitment to supporting those who served extends beyond words—reflected in the opportunities and benefits we provide. Related Content
Military At CommonSpirit Health, we deeply value the sacrifices made by our veterans and their families. Our commitment to supporting those who served extends beyond words—reflected in the opportunities and benefits we provide. Related Content -
 News & Stories at CommonSpirit Health Visit our News & Stories page and learn more about working at CommonSpirit Health, one of the largest nonprofit health systems in the U.S.
News & Stories at CommonSpirit Health Visit our News & Stories page and learn more about working at CommonSpirit Health, one of the largest nonprofit health systems in the U.S. -
 CommonSpirit Health teams up with Proxima Careers CommonSpirit Health and Proxima Careers are partnering to expand access to healthcare careers by providing training, job placement, and ongoing support, helping community members achieve stable, well-paying jobs.
CommonSpirit Health teams up with Proxima Careers CommonSpirit Health and Proxima Careers are partnering to expand access to healthcare careers by providing training, job placement, and ongoing support, helping community members achieve stable, well-paying jobs. -
 National Real Estate Services at CommonSpirit Health Explore career opportunities in real estate strategy, property management, facilities management, design and construction, and support services at CommonSpirit Health's National Real Estate Services team. Article Hiring Process Blog
National Real Estate Services at CommonSpirit Health Explore career opportunities in real estate strategy, property management, facilities management, design and construction, and support services at CommonSpirit Health's National Real Estate Services team. Article Hiring Process Blog -
 CHI Health 2025 Nursing Internship CHI Health Nursing Internship in Nebraska Hiring Process Blog
CHI Health 2025 Nursing Internship CHI Health Nursing Internship in Nebraska Hiring Process Blog -
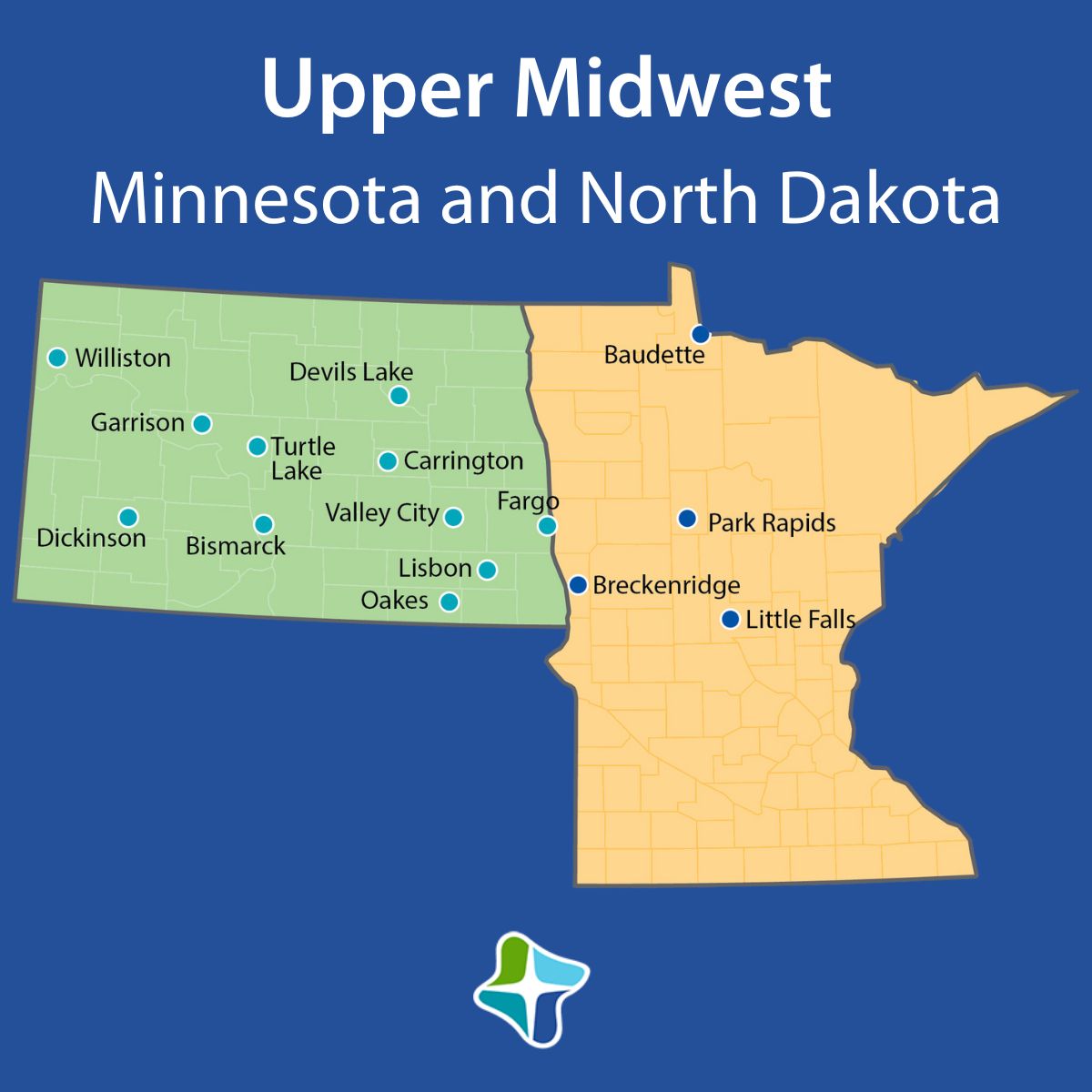
 Upper Midwest Now is the perfect time to join our healthcare team in one of our Upper Midwest locations in Minnesota and North Dakota! Hiring Process Blog
Upper Midwest Now is the perfect time to join our healthcare team in one of our Upper Midwest locations in Minnesota and North Dakota! Hiring Process Blog -
 Radiology Careers at CommonSpirit Health Discover fulfilling career opportunities in Imaging Services at CommonSpirit Health and play a vital role in the diagnosis and treatment of patients' care plans. Hiring Process Blog
Radiology Careers at CommonSpirit Health Discover fulfilling career opportunities in Imaging Services at CommonSpirit Health and play a vital role in the diagnosis and treatment of patients' care plans. Hiring Process Blog -
 Elevate your career as a Surgical Tech with CommonSpirit Health As a surgical technician at CommonSpirit Health, you will play a vital role in guaranteeing that surgical procedures are safe and successful. You will collaborate with leading surgeons and healthcare experts in cutting-edge facilities, providing our patients with the utmost care. Hiring Process Blog
Elevate your career as a Surgical Tech with CommonSpirit Health As a surgical technician at CommonSpirit Health, you will play a vital role in guaranteeing that surgical procedures are safe and successful. You will collaborate with leading surgeons and healthcare experts in cutting-edge facilities, providing our patients with the utmost care. Hiring Process Blog -
 Virtual Command Center Ever wondered what it was like to work in a Virtual Command Center? We’re in the background continuously assessing, reviewing and providing interventions to patients at all hours of the day across the nation. Hiring Process
Virtual Command Center Ever wondered what it was like to work in a Virtual Command Center? We’re in the background continuously assessing, reviewing and providing interventions to patients at all hours of the day across the nation. Hiring Process -
 National Nursing New Grad Residency Program Learn about our new grad nursing program that is nationally-standardized and evidence-based best practice for newly licensed nurses Hiring Process Blog
National Nursing New Grad Residency Program Learn about our new grad nursing program that is nationally-standardized and evidence-based best practice for newly licensed nurses Hiring Process Blog -
 Careers in Environmental Services at CommonSpirit Health Explore career opportunities in Environmental Services at CommonSpirit Health, where we provide a clean and safe environment for patients, staff, and visitors. Hiring Process Blog
Careers in Environmental Services at CommonSpirit Health Explore career opportunities in Environmental Services at CommonSpirit Health, where we provide a clean and safe environment for patients, staff, and visitors. Hiring Process Blog -
 Pursue Your Path as a Medical Assistant at CommonSpirit Health As a Medical Assistant at CommonSpirit Health, you'll be a crucial part of the healthcare system, making a tangible difference in patients' lives. Explore this rewarding career opportunity. Hiring Process Blog
Pursue Your Path as a Medical Assistant at CommonSpirit Health As a Medical Assistant at CommonSpirit Health, you'll be a crucial part of the healthcare system, making a tangible difference in patients' lives. Explore this rewarding career opportunity. Hiring Process Blog -
 CHI Health Career Ladder CNA Program CHI Health has partnered with local Certified Nursing Assistant Programs to offer students C.N.A. training in exchange for full or part-time employment! Hiring Process Blog
CHI Health Career Ladder CNA Program CHI Health has partnered with local Certified Nursing Assistant Programs to offer students C.N.A. training in exchange for full or part-time employment! Hiring Process Blog -
 Meet the CommonSpirit Health Fellows Meet our current CommonSpirit Health Fellows. This is a national, 18-month, system-wide leadership and professional development program that offers a solid foundation for future leaders through hands-on learning experiences, exposure to clinical and non-clinical aspects and mentoring from influential innovators. News/Advancements Blog
Meet the CommonSpirit Health Fellows Meet our current CommonSpirit Health Fellows. This is a national, 18-month, system-wide leadership and professional development program that offers a solid foundation for future leaders through hands-on learning experiences, exposure to clinical and non-clinical aspects and mentoring from influential innovators. News/Advancements Blog -
 On Demand Staffing On Demand Staffing at CommonSpirit Health offers flexible schedules for nurses. News/Advancements Blog
On Demand Staffing On Demand Staffing at CommonSpirit Health offers flexible schedules for nurses. News/Advancements Blog -
 CHI Health Locations Now is the perfect time to join our CHI team in one of our locations! Hiring Process Blog
CHI Health Locations Now is the perfect time to join our CHI team in one of our locations! Hiring Process Blog -
 CommonSpirit is Advancing Equitable Opportunities for Hiring and Training CommonSpirit Health is engaged in the Healthcare Anchor Network’s Impact Workforce Commitment, an initiative to hire and train individuals who may have faced employment barriers due to low income, lack of access to education and training, and other socioeconomic challenges. Hiring Process Blog
CommonSpirit is Advancing Equitable Opportunities for Hiring and Training CommonSpirit Health is engaged in the Healthcare Anchor Network’s Impact Workforce Commitment, an initiative to hire and train individuals who may have faced employment barriers due to low income, lack of access to education and training, and other socioeconomic challenges. Hiring Process Blog
Equal Opportunity
CommonSpirit Health™ is an Equal Opportunity/Affirmative Action employer committed to a diverse and inclusive workforce. All qualified applicants will be considered for employment without regard to race, color, religion, sex, sexual orientation, gender identity, national origin, age, disability, marital status, parental status, ancestry, veteran status, genetic information, or any other characteristic protected by law. For more information about your EEO rights as an applicant, please click here [PDF].
CommonSpirit Health™ will not discharge or in any other manner discriminate against employees or applicants because they have inquired about, discussed, or disclosed their pay or the pay of another employee or applicant. However, employees who have access to the compensation information of other employees or applicants as a part of their essential job functions cannot disclose the pay of other employees or applicants to individuals who do not otherwise have access to compensation information, unless the disclosure is (a) in response to a formal complaint or charge, (b) in furtherance of an investigation, proceeding, hearing, or action, including an investigation conducted by the employer, or (c) consistent with the contractor’s legal duty to furnish information. 41 CFR 60-1.35(c). External hires must pass a post-offer, pre-employment background check/drug screen. Qualified applicants with an arrest and/or conviction will be considered for employment in a manner consistent with federal and state laws, as well as applicable local ordinances, ban the box laws, including but not limited to the San Francisco and Los Angeles Fair Chance Ordinances. If you need a reasonable accommodation for any part of the employment process, please contact us by telephone at (415) 438-5575 and let us know the nature of your request. We will only respond to messages left that involve a request for a reasonable accommodation in the application process. We will accommodate the needs of any qualified candidate who requests a reasonable accommodation under the Americans with Disabilities Act (ADA). CommonSpirit Health™ participates in E-Verify.